Pharma Competency
Tekpak is a Trusted Packaging Line Automation Partner to many of the largest Pharmaceutical Companies in the world.
Our projects are managed using the Agile Project Management SCRUM Method. From Project Kick-Off to Design Qualification, Fabrication, FAT, Installation and SAT Handover our projects operate in 2-4 week sprints. Clear objectives and milestones allow our agile project teams to respond quickly and efficiently which reduces lead times allowing our machines to go into full production more quickly.

Design for GMP
Tekpak machines are designed with a GMP philosophy. This means that the machines are hygienic by design and the quality and integrity of the product, packaging and data are maintained throughout the machine processes.
We take great care during the design process to ensure the requirements of the Industries that we serve are met.
We understand that our clients operate in a Good Manufacturing Process environment and that there are certain pre-requisites for machinery in this environment.
GMP Design Features
1. Hygienic by Design
Maximum smooth surfaces and minimal holes and crevasses is achieved by careful Mechanical Design of machine frames, structural support, fixings, brackets, pneumatic piping, electrical cable routing etc.
2. Easy to Clean
We take great care to ensure the machines are designed for ease of access and cleaning. Materials are selected, which can be cleaned using standard cleaning agents such as 70% Ethanol and using the standard wipe down technique.
3. Line Clearance
There are no hidden zones for product to dwell or be unseen. Operator Interfaces have a Line Clearance Mode which reduces the time for operators to clear the line of product.
4. Product Quality and Integrity Maintained
There is positive control of the product throughout the process so that there is no impact on product quality and integrity. Components are selected which have minimal impact on the product, such as pneumatic devices with cushioning and pressure limiting, bearings which are sealed and with lifelong lubrication, materials selected which do not shed or mark the product.

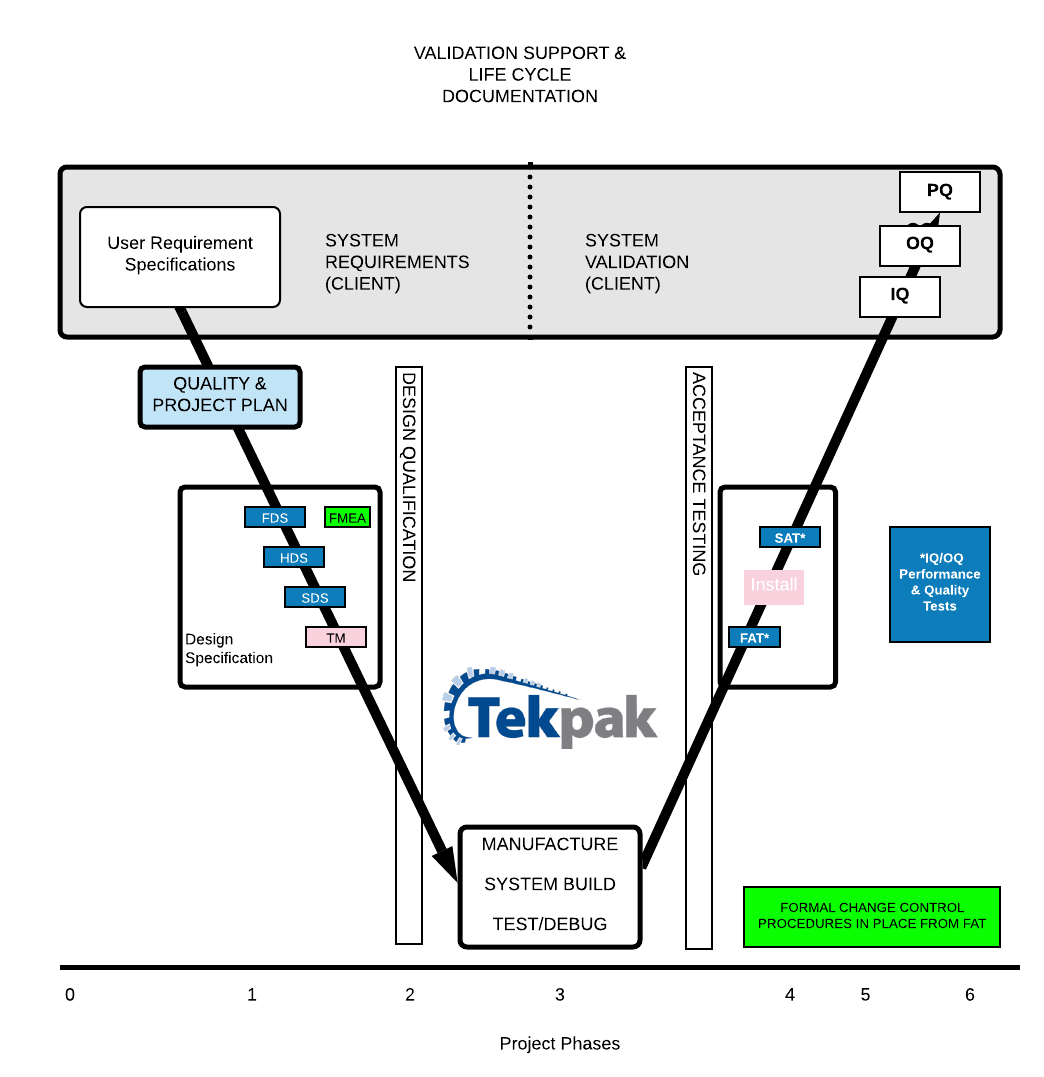
Validation Support
We take great care to understand the requirements of our clients through a thorough analysis of their URS. Where the URS is still in development, we can assist the client to finalise their technical specifications and requirements.
Our projects are executed using GAMP 5 methodology, following a Quality and Project Plan. The full project lifecycle is documented from Design Specifications FDS/HDS/SDS and Design Qualification to Testing, Approval, FAT and SAT.
1. Project Kick off
Kick-off is a vital phase in the project. Tekpak has an Internal Technical Project Kick-Off meeting to develop the Design Plan, identify the risks and milestones on the project. There will also be a Project Kick-Off meeting with the client project team to introduce all of the project stakeholders, agree on the schedule and clarify any open items in the URS.
2. Design Qualification
The detailed design phase of the project is where a thorough analysis of the User Requirements takes place. The Design is Risk Assessed (FMEA and Safety Risk Assessment) and the Design Specification documents are developed (FDS/HDS/SDS) as working drafts and ready for sign off at DQ with the client.
3. System Build
The longest phase in the project starts with the ordering of long lead time items and ends at Pre-FAT. This phase of the project incorporates procurement, fabrication, assembly, programming and debugging. During which time there is regular communication and status updates with the client and Design Specifications are finalised.
4. System Test & Approval
We have the capability, facilities and experience to run complete line FAT at Tekpak. In many instances, we incorporate OEM equipment to form a full line test and integration at Tekpak before installation at the client site. FAT includes IQ/OQ, Performance and Quality Tests.
5. System Handover & Training
After a successful SAT, the client personnel are trained to run the equipment through the Validation process. SAT includes IQ/OQ, Performance and Quality Tests. Any tests that could not be performed at Tekpak are also completed during this time (for example, those which rely on continuous production or other client-specific conditions).
6. Validation Support
In most cases, the client will complete their own IQ/OQ/PQ after SAT with remote support from Tekpak.
7. Go Live!
Tekpak will be available for Go Live Support should this be required.

21 CFR PART 11
We have developed a deep understanding of the needs of our multi-national clients, in particular those in the pharmaceutical sector. Our project and Design teams all receive training regarding the regulatory requirements.
Our machines can be configured to assist with 21CFR Part 11 compliance. Our control systems can be specified to provide Audit Log, Time Synchronisation, Active Directory password protection, User Groups and permissions.
Communication Protocols
Our machines can be provided with Industry 4.0 ready options and can communicate with other devices and higher-level SCADA, MES and OEE systems using defined protocols such as OPC UA, LDAP, Ethernet/IP, Profinet, I-O Link, Modbus, TCP/IP etc.


