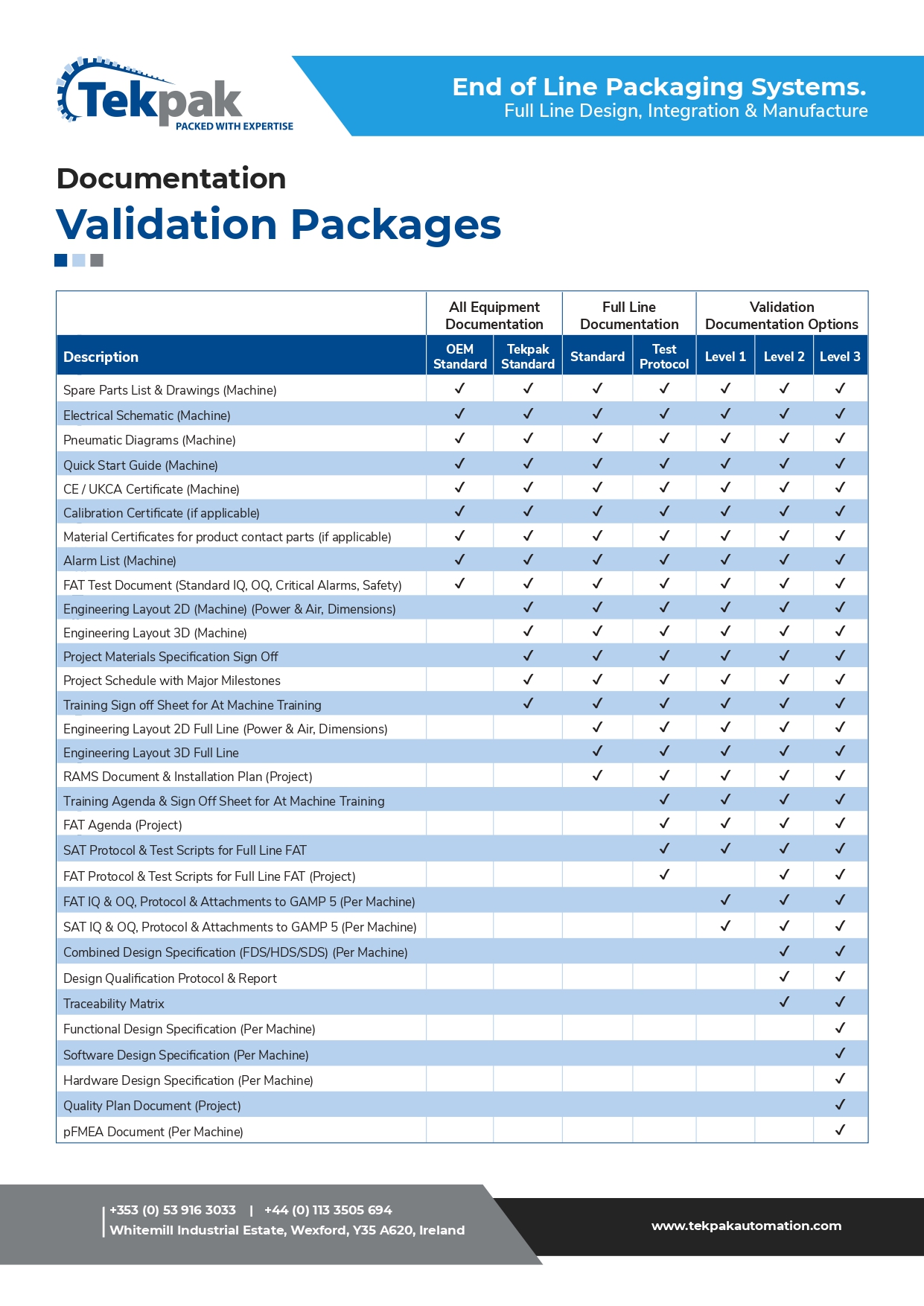
Validation & Documentation
Tekpak offers comprehensive validation services to minimise business risks, stabilise manufacturing processes, and enhance product safety. Our expert team specialises in the pharmaceutical, biotechnology, and medical devices industries, mastering both traditional and agile processes. Flexible, efficient, and scalable, our user-focused solutions cater to companies of all sizes.
Full Validation Support
We take great care to understand the requirements of our clients through a thorough analysis of their URS. Where the URS is still in development, we can assist the client to finalise their technical specifications and requirements. Our projects are executed using GAMP 5 methodology, following a Quality and Project Plan. The full project lifecycle is documented from Design Specifications FDS/HDS/SDS and Design Qualification to Testing, Approval, FAT and SAT.
Our projects are managed using the Agile Project Management SCRUM Method. From Project Kick-Off to Design Qualification, Fabrication, FAT, Installation and SAT Handover our projects operate in 2-4 week sprints. Clear objectives and milestones allow our agile project teams to respond quickly and efficiently which reduces lead times allowing our machines to go into full production more quickly.
Validation Support
We take great care to understand the requirements of our clients through a thorough analysis of their URS. Where the URS is still in development, we can assist the client to finalise their technical specifications and requirements.
Our projects are executed using GAMP 5 methodology, following a Quality and Project Plan. The full project lifecycle is documented from Design Specifications FDS/HDS/SDS and Design Qualification to Testing, Approval, FAT and SAT.

Full Validation Support
Project Kick-Off
The kick-off phase is a crucial stage of the project. Tekpak begins with an internal technical project kick-off meeting to develop the design plan, identify risks, and outline project milestones. A separate project kick-off meeting is held with the client’s project team to introduce all stakeholders, agree on the schedule, and address any open items in the URS.
Design Qualification (DQ)
During this detailed design phase, the User Requirements are thoroughly analysed. Risk assessments are conducted (FMEA and Safety Risk Assessment), and the Design Specification documents (FDS/HDS/SDS) are developed as working drafts. These documents are finalised and signed off at the Design Qualification stage with the client.
System Build
The longest project phase begins with the procurement of long lead-time items and concludes at Pre-FAT. This stage includes procurement, fabrication, assembly, programming, and debugging. Regular communication and status updates are provided to the client, and the Design Specifications are finalised during this time.
System Test & Approval
Tekpak has the facilities, expertise, and experience to conduct complete line FATs, often incorporating OEM equipment to create a full-line test and integration. FAT includes IQ/OQ, performance, and quality tests, ensuring comprehensive validation before installation at the client site.
System Handover & Training
Following a successful SAT, client personnel are trained to operate the equipment through the validation process. SAT includes IQ/OQ, performance, and quality tests, with any tests that could not be performed at Tekpak (e.g., those reliant on continuous production or specific client conditions) completed at this stage.
Validation Support
In most cases, clients conduct their own IQ/OQ/PQ after SAT, with remote support from Tekpak to ensure a seamless validation process.
Go Live!
Tekpak provides support during the Go Live phase, ensuring smooth implementation and resolving any issues as needed.
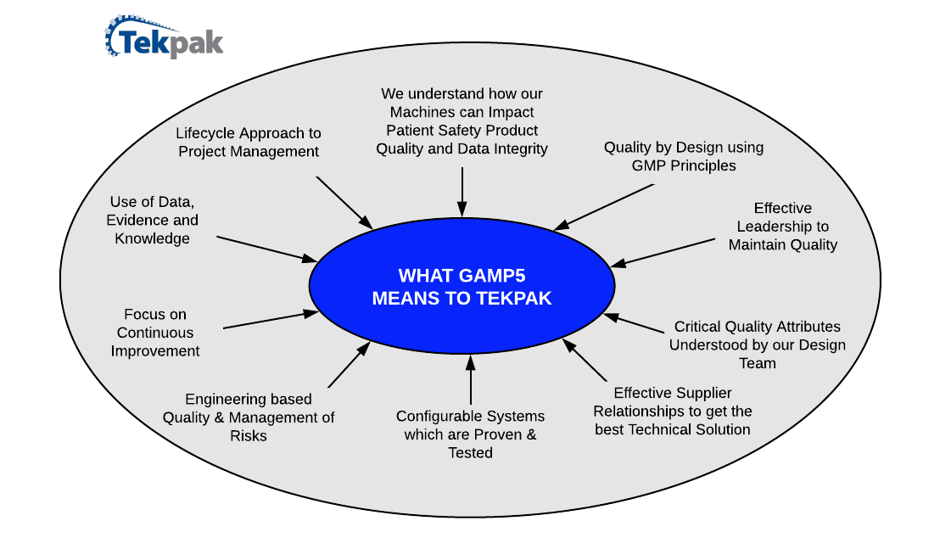
Regulatory Challenges Support
Risk management using a risk-based validation approach.
Analysis of the Quality Management System (QMS) with a focus on validation processes.
Development of a validation plan defining project scope and validation strategy.
Execution of Computer System Validation (CSV), including equipment and system qualification.
GAMP®5-based solutions with the V-model or tailored, customer-specific compliance procedures.
Creation of Standard Operating Procedures (SOPs) and training programmes.
Expert consulting on data integrity.
Comprehensive audit management and support.
Assistance with validation of cloud solutions, including Infrastructure as a Service (IaaS), Platform as a Service (PaaS), and Software as a Service (SaaS).
Our experts are ready to support you on-site!